| What did he compose for the model? | What did he also discover? | What was he the first to do? |
| Matter was invisible with distinct masses | Discovered that matter was invisible with distinct masses | Develop a full atomic theory model in 1803 |
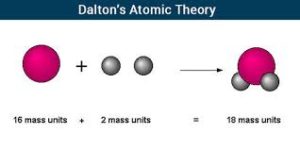

John Dalton contributed to the Atomic theory by composing that matter was invisible with distinct masses and different properties, and also figured out that all atoms are also indestructible. He was actually one of the first chemists to propose a fully developed atomic theory in 1803. He had a second theory which was that all atoms of the given element were all identical in mass and property. Most of these theories he made weren't new but they were improved ideas from other scientists like Joseph-Louis Proust and Henry Cavendish. His idea lead to the modern periodic table in which we still use today. His theory was still used later on but changed by chemists to add subatomic particles and isotopes. Click on their image to go to their Wikipedia page.
Go Back