| Used what chemists wave theory? | Difference between his and Bohr's model? | Created what kind of model? |
| de Broglie's matter wave theory | His model had electrons moving but not in orbit. | Created a mathematical model. |

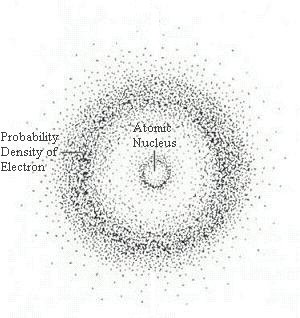

Schrodinger was a chemist just like Bohr and created an accurate model of the atom. He used de Broglie's matter wave theory as his base to develop an accurate model of the atom. He showed that electrons don't follow in defined orbits unlike Bohr's model, but are found in orbitals. He created a mathematical model for electrons by using the work of de Broglie and combining it with his own equation. Schrodinger also formulated a wave equation that accurately calculated the energy levels of electrons in an atom. Schrodinger's atomic model is on the quantum mechanical and wavelength features of the electrons. Some of the work that Schrodinger did was shown in the work of Niels Bohr. Click on their image to go to their Wikipedia page.
Go Back